
Manganese Superoxide Dismutase
Manganese Superoxide Dismutase (MnSOD)
Background
Ionizing radiation in space is a very prominent health hazard to astronauts and is deadly during prolonged space travel, such as planned trips to Mars. Radiation is a significant source of reactive oxygen species (ROS), which cause major health problems even for the average person on Earth. Excessive ROS create oxidative stress, which is involved in the etiology of cancer, neurological disorders, heart disease, etc.
Superoxide dismutases (SODs) are enzymes that provide the first line of defense against reactive oxygen species (ROS). SODs scavenge two superoxide molecules and allow conversion into regular oxygen and hydrogen peroxide through a cyclic redox fashion utilizing a catalytic metal ligand (Manganese is this case). Despite the biological importance of SODs, the complete enzymatic reaction is unknown due to limitations in identifying the binding sites of the substrate, hydrogens, and products.
One method that actually allows for the visualization of active site in MnSOD to gain a more complete understanding of the chemical reactions that occur. is neutron diffraction. Neutron diffraction requires large perfect crystals (greater than 1mm3). It also requires substitution of all of the hydrogens with deuteriums. The reason for this is that deuterium has a neutron cross section to other atoms that is close in magnitude to the other atoms that make up the protein.
Perdeuterated Protein Samples
Protein crystallization requires large quantities of pure protein (several grams) compared to more commonly used protein assays (μ grams). Crystals created for neutron diffraction need the hydrogens substituted with deuterium. Proteins are normally grown in recombinant cells that have been engineered to over express the protein of interest in a culture. An added difficulty in producing proteins for neutron diffraction is that ideally all of the hydrogens need to be substituted with deuterium. This process is both expensive and complicated requiring all of the reagents to be deuterium substitutions as well. Fortunately researchers who receive time at the Spallation Neutron Source on Oakridge National Lab campus also get access to the Bio-Deuteration Laboratory which aid researchers in translating their expressions systems into perdeuterated versions.
Microgravity Crystallization on the International Space Station
Neutrons interact weakly with matter. This means that for neutron diffraction experiments the crystals must be both large (>1mm3) and perfect (no defects) to maximize the signal to noise. One of the best ways to grow these types of crystals is to use the International Space Station because the microgravity environment has been observed to produce crystals that are both large and close to perfect based on earlier experiments. Over the years several different experimental setups have been tested. One of the simplest and most reliable is counter diffusion with capillaries stored in a Granada crystallization box.
Our ISS mission page can be found HERE.
Counter Diffusion Crystallization
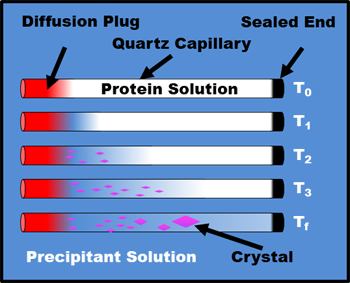
Counter Diffusion Schematic
Granada Crystallization Box
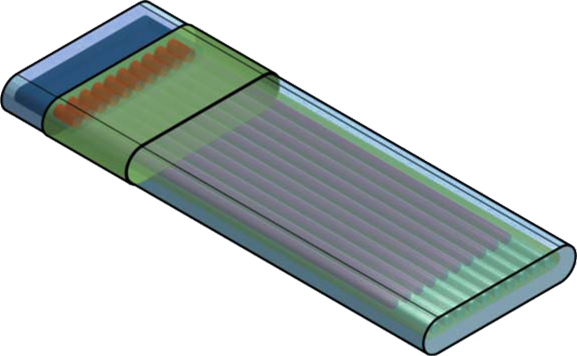
Granada Crystallization Box
Crystallization Assembly
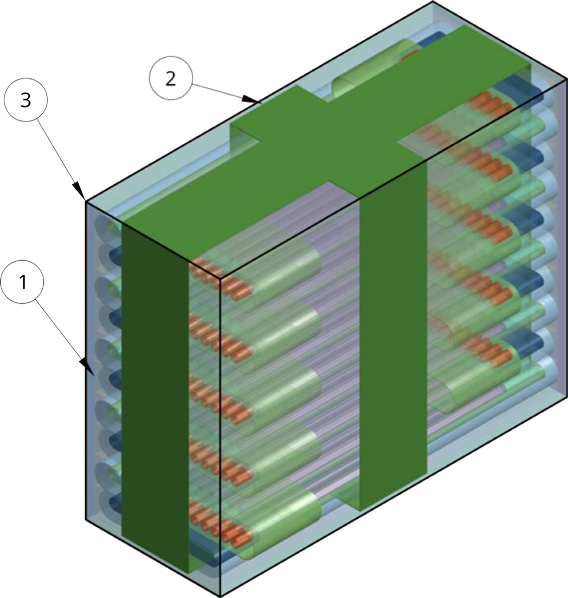
UNMC Supplied Crystallization Assembly
PC-GEOB
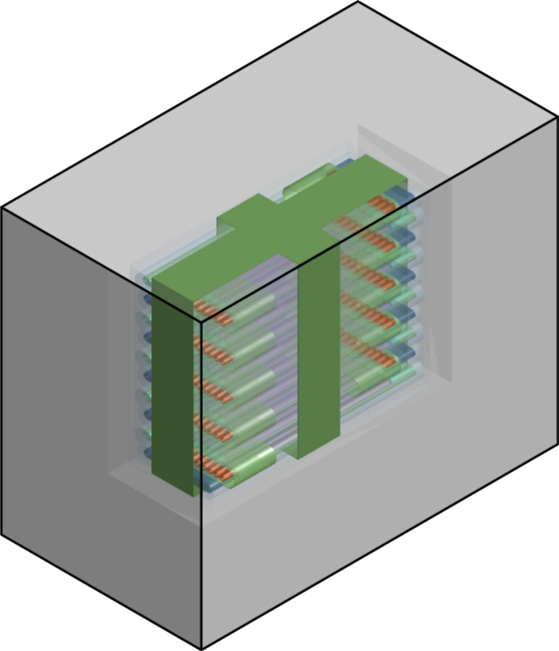
PC-GEOB Schematic

PC-GEOB
Experiment on the ISS

μg Experiment Flight Plan
Perfect Crystals Mission
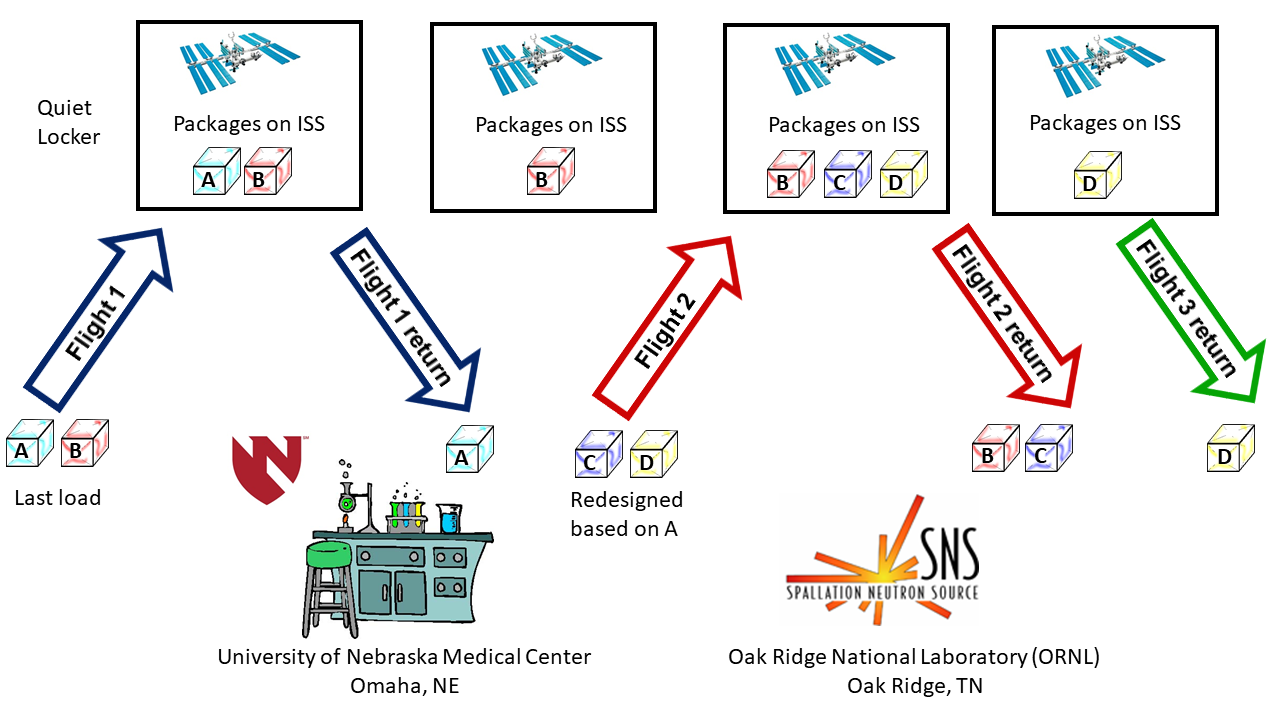
μg Experiment Flight Plan
Perfect Crystals Flight Details
| Date | Event | Description |
|---|---|---|
| 11/27/2018 | SpaceX 16 (CRS) | PC-GEOBs A&B will be delivered to the ISS. |
| 01/09/2019 | SpaceX 16 (CRS) | PC-GEOB A will be returned to Earth. Initial results will be used to improve PC-GEOBs C&D. |
| 07/25/2019 | SpaceX 18 (CRS) | PC-GEOBs C&D will be delivered to the ISS. |
| 08/27/2019 | SpaceX 18 (CRS) | PC-GEOB B and C returned to Earth. |
| 01/07/2020 | SpaceX 19 (CRS) | PC-GEOB D returned to Earth. |
Neutron Diffraction
A common approach to determine protein and other atomic structures at very high resolutions is X-Ray diffraction. X-Ray diffraction requires growing the sample as a crystal and then placing the crystal in an xray beam and recording the diffraction pattern as the sample is rotated. A computer can be used to determine the electron density that created the diffraction pattern. Atomic models can be fit to the electron density to solve the structure. The maximum resolution that can be resolved by an imaging system is equal to 1/2 the wavelenght used by the imager. A common wavelenght for x-rays is 1.54 Angstroms which gives a maximum resolution of 0.77 Angstroms. For a good quality experimental setup and high quality crystals individaul atoms can be observed. Each type of atom has a scattering cross section that determines how visible this type of atom is relative to the other atoms making up the sample. Ideally all of the atoms should have the same scattering factor. For macromolecules the most common atoms are carbon, nitrogen, oxygen and hydrogen. For x-rays the scattering factor for hydrogen is so small that in a x-ray diffraction experiment hydrogens are not visible. Fortunately, is possible to use subatomic particles as a substitute for electromagnetic radiation. For macromolecules neutrons can be used in the diffraction experiment. Neutrons have a vastly different scattering cross section where the relative sizes of carbon, nitrogen, oxygen and deuterium are almost the same. Deuterium is a stable form of hydrogen with one neutron in the core of the atom but it is also rare. The figure below shows the scattering cross sections for atoms in x-rays and neutrons. The large signal for hydrogen is a bit missleading as the scattering is uncorellated for neutrons which causes the signal to be corrupted with noise which is the primary reason why the hydrogens need to be replaced with dueterium to get the best signal to noise in these types of experiments. An example below demonstrates the ability to "see" the hydrogens using neutrons vs x-rays. Oak Ridge National Lab operates the Spallation Neutron Source which has the MaNDi beamline that is dedicated to neutron diffraction of macromolecules.
Scattering Cross Sections
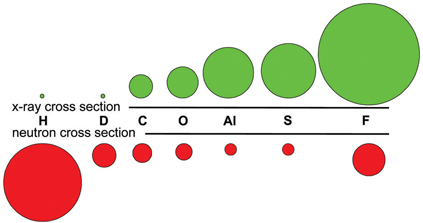
X-ray vs. Neutron Scattering Cross sections
X-Ray vs. Neutron Electron Density
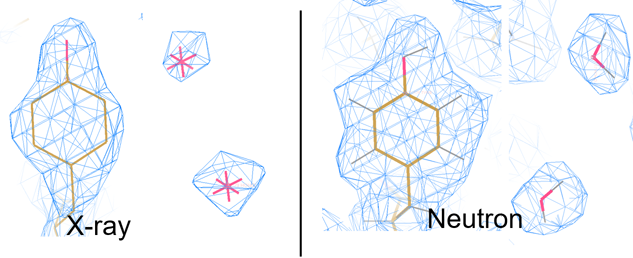
X-ray vs. Neutron Scattering Cross sections
Spallation Neutron Source (SNS) at Oak Ridge National Lab

Spallation Neutron Source
MANDi Beamline
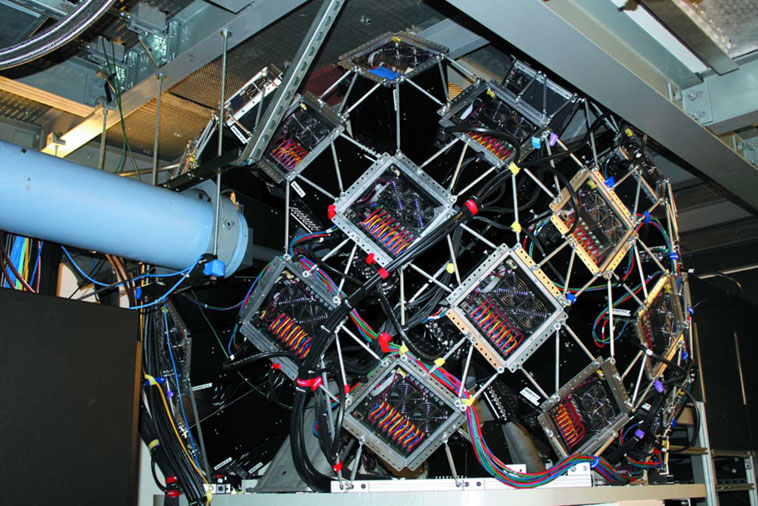
Detector at the MANDi Beamline
Neutron Diffraction Image
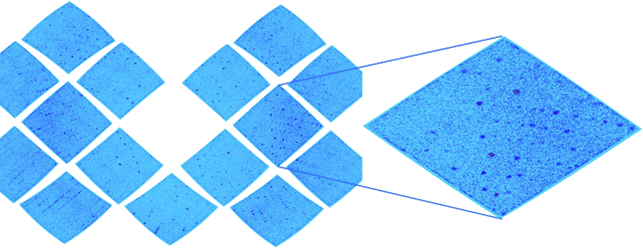
MnSOD Crystal Diffraction
